News
How does protein degradation regulate plant immune signalling?
The Spoel Research Group publishes a comprehensive review paper in Frontiers in Plant Science on the role of Cullin-RING (CRL) ubiquitin ligases and the proteasome in salicylic acid-mediated plant immune signalling.
Edinburgh, March 13, 2015 - Plant immune responses against biotrophic pathogens are regulated by the signalling hormone salicylic acid (SA). SA establishes immunity by regulating a variety of cellular processes, including programmed cell death (PCD) to isolate and kill invading pathogens, and development of systemic acquired resistance (SAR), which provides long-lasting, broad-spectrum resistance throughout the plant.
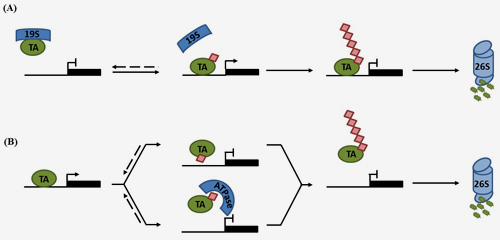
Central to these processes is post-translational modification of SA-regulated signalling proteins by ubiquitination, i.e. the covalent addition of small ubiquitin proteins. Emerging evidence indicates SA-induced protein ubiquitination is largely orchestrated by Cullin-RING ligases (CRLs), which recruit specific substrates for ubiquitination using interchangeable adaptors. Ligation of ubiquitin chains interlinked at lysine 48 leads to substrate degradation by the 26S proteasome.
In a Frontiers in Plant Science review paper the Spoel Research Group now discuss how CRL-mediated degradation of both nucleotide-binding/leucine-rich repeat domain containing (NLR) immune receptors and SA-induced transcription regulators are critical for functional PCD and SAR responses, respectively. By placing these recent findings in context of knowledge gained in other eukaryotic model species, potential alternative roles for processive ubiquitination in regulating the activity of SA-mediated immune responses are highlighted.
Related links:
Furniss JJ and Spoel SH (2015) Cullin-RING ubiquitin ligases in salicylic acid-mediated plant immune signaling. FRONTIERS IN PLANT SCIENCE 6:154 (PDF).